Helping YOU Engineer Safe, Effective, Efficient, and Satisfying
Medical Devices and Health Information Systems
We are a healthcare engineering private practice focused on helping you develop & deploy medical devices and health
information systems that are safe, effective, efficient to produce & maintain, and satisfying to all your stakeholders. Our
principals, both of whom are professional members of the American Society of Safety Professionals, provide highly
personalized service tailored to your specific requirements and constraints. We have been serving domestic and
international clients since 1997. Our clients have ranged from global multi-nationals to new startups, as well as
numerous regulatory and technical consulting firms.
We have experience with a very broad range of medical devices - from accessories for Class 1 devices to untried Class
3 medical devices - as well as serving as expert witnesses in medical device product liability litigation. It is our belief
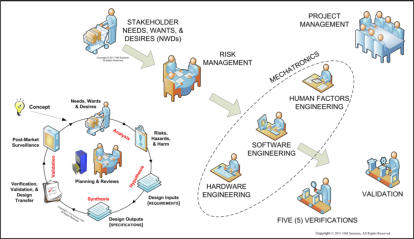
that the best defense against medical device product liability is a structured, systematic, and well-documented product
development process compliant with FDA’s Quality System regulations, ISO 13485/14971, and IEC 62304/62366.
For a broad overview of the journey from the bench to the medical device market place, please see “US Medical Device
Innovation: Moving from the Bench to Market” presented at the 2012 IEEE Healthcare Innovation Conference:
Translational Engineering in Health & Medicine.
For a short tutorial on evaluating whether your innovation is commercially viable, try Prof. Herzlinger’s recent article:
“Tutorial: Is Your Health Care Innovation Commercially Viable?”
We provide: • medical device regulatory assistance with 510(k)s (& e-Copy), IDEs, PMAs, 483s, and MDRs; • technical and management assistance with the development and implementation of FDA-compliant quality management systems and ISO14971 - compliant risk analysis & risk management; • assistance with "cradle-to-grave" human factors & ergonomics engineering, including usability & user experience studies; • technical and management assistance with the design, development, and statistically-valid verifications and validations of mechatronic medical devices; and • complete confidentiality and personal service for all our clients. We are particularly interested in assisting you in the development, deployment, and maintenance of highly safety-critical medical devices and health information systems. Please browse our website for more information. Let us know how we and our colleagues may help you. We are always happy to spend 30-60 minutes chatting with you to ensure that we can assist you. Please don't forget to check out our e-Library.









Copyright © 2012-20 Samaras & Associates, Inc., All Rights Reserved
Professional Workshops & Presentations
• For a portfolio of essays on medical device product development, please click here. (Requires Flash; will not work on most i-Devices) • QMED Focus on Fundamentals 3-day Webinar on ”How to Manage Risk Throughout Medical Device Product Development Cycle and Beyond”. To access the recorded audio and slide decks, register here. • HFES 2016 Risk Management Workshop, Washington, DC. September 18, 2016.