Copyright © 2012-20 Samaras & Associates, Inc., All Rights Reserved
Human Factors & Ergonomics
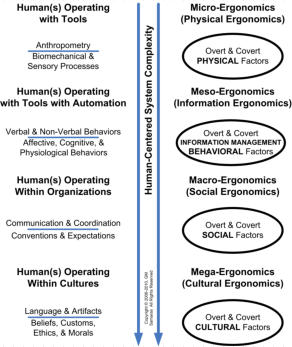
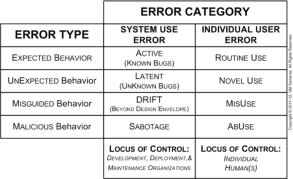
OUR SERVICES: We can assist you with all aspects of human factors and ergonomics (HF&E) design & analysis of your medical device or health information system. We use standard tools (e.g., function & task analysis software), as well as proprietary analytic tools and templates to conduct efficiently a comprehensive HF&E analysis of your specific medical device or information system, its intended user(s) and intended use environment(s). We then use this to assist you in your HW, SW, and labeling design, risk management, and testing efforts. We also can help you design, execute, analyze, and document HF&E validation studies, including labeling studies, usability studies, and clinical use trials. OUR PRINCIPALS: One of our principals is a board-certified human factors engineer with extensive medical device design & analysis experience. The other is one of a very few clinicians board-certified in human factors, with extensive human factors experience and real-world experience using medical devices and healthcare information systems. System Use and user errors may be the last bastion of equipment and process safety and effectiveness problems. The source of use and user errors can be the result of problems with equipment development, deployment, and maintenance and/or can be the result of problems with user training, work structure, and the work environment (see upper figure on the right and a recent article by one of our principals describing 3 case studies). While it was previously thought that design problems could be alleviated with labeling and training, this is now generally recognized not to be the case. The discipline of human factors & ergonomics (HF&E) has a body of knowledge to deal with the interaction of humans and their artifacts and with the design of systems in which people participate. HF&E engineering is fundamentally about developing, deploying, and maintaining products, processes, and services for human use. The discipline can be roughly divided into sets of 4 major areas (see middle figure on right): physical ergonomics, information management ergonomics, social ergonomics, and cultural ergonomics. For greater detail, please see recent article by both of our principals. HF&E engineering considerations participate in a manner similar to hardware, software, and economic considerations in the development of requirements, in compliance with appropriate regulations and standards, and in the engineering of system reliability and system safety & security (please refer to middle figure on mechatronics page). HF&E considerations are important at all stages of the system lifecycle (development, deployment, maintenance, and disposal). Two critical activities for medical device developers are: “formative” evaluations (design verifications; 21 CFR 820.30f) and “summative” evaluation (design validation; 21 CFR 820.30g). The FDA requires specific HF&E design documentation, as shown in the lower figure on the right. The current FDA Guidance and two relevant American National Standards are: • Applying Human Factors and Usability Engineering to Medical Devices: Guidance for Industry and Food and Drug Administration Staff, April 3, 2016 • ANSI/AAMI/IEC 62366-1:2015, Medical Devices - Part 1: Application of usability engineering to medical devices • ANSI/AAMI HE75:2009 (R) 2013, Human factors engineering - Design of medical devices. HF&E engineering is not merely about usability or user experience, although these are important factors. "Cradle to grave" HF&E engineering addresses not only physical, behavioral, social, and cultural factors of the intended user(s) (e.g., clinicians, BMETs, managers, etc.) in the intended use environment(s), but also addresses the HF&E issues during development (e.g., integration of hardware, software, and human factors design and testing) and during deployment (e.g., selection, installation, maintenance, and disposal). Please refer to Figure 5 in a recent article by one of our principals on avoiding system use errors during development and deployment.