










Copyright © 2012-20 Samaras & Associates, Inc., All Rights Reserved
FDA Regulatory Assistance
In the US, the marketing of medical device innovations are regulated by the Food and Drug Administration's Center for Devices and Radiological Health (CDRH) in Silver Spring, Maryland. Regulation is risk-based and three risk classifications are identified: Class 1 (low risk; FDA registration & listing), Class 2 (moderate risk; FDA premarket notification), and Class 3 (high risk; FDA premarket approval). Risk depends primarily upon intended use and the uncertainty regarding establishing safety and effectiveness of a new medical device. The figure on the right, based on the well-known 1990 Henderson-Clark product innovation model, describes this in greater detail for Class 2 and 3 devices. New devices that have a legally marketed predicate are required to provide premarket notification and obtain administrative clearance prior to marketing in the US. New devices that have no legally marketed predicate are required to obtain premarket approval (which is very different from administrative clearance) prior to marketing in the US. Clinical trials are required for all premarket approval requests and for a minority of premarket notification requests. We can assist you with: • obtaining FDA small business certification for lower cost user fees; • completing/maintaining your establishment registration and device listing(s); • preparing/submitting a request for a device classification ruling, also known as a 513(g); • preparing/submitting Traditional, Abbreviated, or Special premarket notifications, also known as a 510(k) and the now mandatory e-Copy; • preparing/submitting a request for an Investigational Device Exemption (IDE) and then preparing/submitting a study proposal, including an Informed Consent compliant with 21 CFR Part 50, for Institutional Review Board (IRB) approval; please note that an IDE is NOT an exemption from Design Controls (21 CFR 812.1(a)); • preparing, justifying, and submitting a request for a de novo reclassification, if your device is found to be Not Substantially Equivalent (NSE) to your claimed predicate; • preparing/submitting a request for premarket approval (PMA) and then post-PMA supplements; • preparing/responding to FDA District Office inspection deficiencies, also known as Form 483 observations; • preparing/submitting reports under the Medical Device Reporting (MDR) rule; and • various other medical device regulatory services. Please see next sections for assistance with: Design Controls, Risk Management, & Human Factors. They are all interconnected!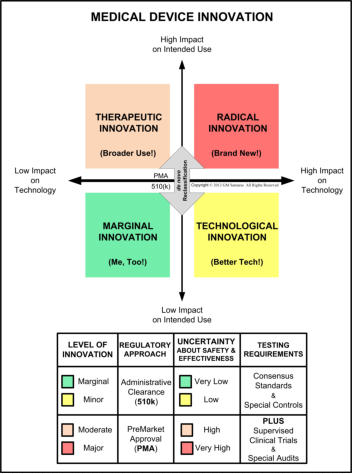
Keys to rapid, successful 510(k) clearance:
•
correctly identifying one (or more) legally marketed
predicate(s),
•
correctly identifying the required testing for your
specific device, as specified in (international)
consensus standards and FDA special controls,
•
correctly and completely doing the required testing
and testing documentation,
•
preparing a submission that includes all the
requisite elements for your type of device, and
•
preparing the now mandatory, validated e-Copy.






























