










Copyright © 2012-20 Samaras & Associates, Inc., All Rights Reserved
Mechatronic Devices
OUR SERVICES: We have extensive experience with medical device development HW, SW, HF&E and Quality engineering, in addition to a practical understanding of the clinical application of a wide spectrum of medical devices. We use custom checklists and proprietary tools and templates to help you efficiently assess the quality and completeness of HW, SW, and HF&E documentation (required for submission to the FDA), the degree of integration between the disciplines, and the management activities to increase mechatronics maturity in your (or your subcontractor’s) firm. OUR PRINCIPALS: One of our principals is a licensed professional engineer (both electronics and software engineering) and former medical school professor with a doctorate in engineering management, multiple medical device patents, and 25+ years experience with embedded software development. The other is a clinician with real-world experience using medical devices and healthcare information systems. An important historical innovation in healthcare has been the advent
of mechatronic medical devices - devices that integrate sensor and
effector mechanical and electrical hardware (HW) with information-
driven software (SW) processes into a potentially synergistic whole,
offering increasingly sophisticated functionality. They range the
gamut from simple positioning systems, to infusion pumps, to
robotic surgical devices, healthcare information technologies
(including medical device data systems) and in vitro diagnostic
device (IVD) instruments.
While the use of mechatronics offers enormous potential for
increasingly sophisticated functionality, it also presents equally large
quality problems with interdisciplinary development, deployment,
utilization, and maintenance. These are not merely technical issues
(e.g., promoting integration of HW and SW development by
automatically generating a new hardware abstraction layer with
each HW revision), but also organizational issues (e.g., preventing
development from occurring in independent silos) and project
management issues (e.g., emphasizing and prioritizing quality
milestones over schedule and budget milestones). How an
organization deals with the HW, SW, and all-encompassing human
factors & ergonomics (HF&E) issues may be viewed as a measure
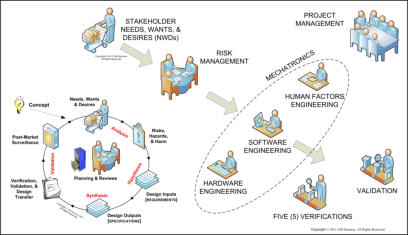
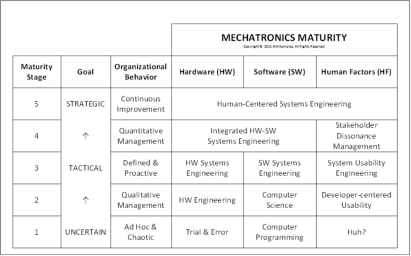
of medical device mechatronics maturity (see upper figure on the
right and recent article by one of our principals).
Mechatronics maturity may be defined as: a measure of how good
you are at avoiding or recovering from the creation or propagation
of System Use errors. Testing (verifications and validation; click to
see diagram) is an outcomes approach to quality; it is necessary,
but not sufficient. Quality engineers understand this well!
Inspection alone historically has proven inadequate and, in the 20th
century, quality management moved successively through statistical
quality control, quality assurance, and strategic quality
management. This is the process approach to quality practices; it is
a management strategy that realizes that outputs are inextricably
linked to inputs and transformations. The specific processes used
by medical device development organizations can help estimate
their mechatronics maturity ... and that is a predictor of new product
development (NPD) quality, which is one reason why the FDA
believes that quality system management audits have value.
Mechatronic medical devices are the result of integration of the
engineering work of various HW disciplines (mechanical, electrical,
etc.), various SW disciplines (embedded, application, etc.), and - of
necessity, as many are now beginning to realize - various HF&E
disciplines (micro-, meso-, macro-, and mega-ergonomics; please
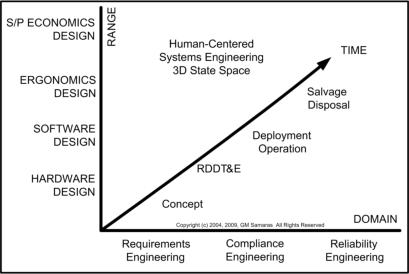
refer to diagram for definition of terms). Please also refer to middle
and lower figures on the right. Practitioners of these various
disciplines speak different technical languages and have very
different perspectives of the engineering process; in many cases,
some of the SW and HF&E practitioners have no engineering
background. This creates significant engineering management
problems that are typically not resolved with standard project
management methods. Some pretend that these are problems with
the HW, SW, and HF&E practitioners; in fact, they are engineering
management problems!
An important historical innovation in healthcare has been the advent
of mechatronic medical devices - devices that integrate sensor and
effector mechanical and electrical hardware (HW) with information-
driven software (SW) processes into a potentially synergistic whole,
offering increasingly sophisticated functionality. They range the
gamut from simple positioning systems, to infusion pumps, to
robotic surgical devices, healthcare information technologies
(including medical device data systems) and in vitro diagnostic
device (IVD) instruments.
While the use of mechatronics offers enormous potential for
increasingly sophisticated functionality, it also presents equally large
quality problems with interdisciplinary development, deployment,
utilization, and maintenance. These are not merely technical issues
(e.g., promoting integration of HW and SW development by
automatically generating a new hardware abstraction layer with
each HW revision), but also organizational issues (e.g., preventing
development from occurring in independent silos) and project
management issues (e.g., emphasizing and prioritizing quality
milestones over schedule and budget milestones). How an
organization deals with the HW, SW, and all-encompassing human
factors & ergonomics (HF&E) issues may be viewed as a measure
of medical device mechatronics maturity (see upper figure on the
right and recent article by one of our principals).
Mechatronics maturity may be defined as: a measure of how good
you are at avoiding or recovering from the creation or propagation
of System Use errors. Testing (verifications and validation; click to
see diagram) is an outcomes approach to quality; it is necessary,
but not sufficient. Quality engineers understand this well!
Inspection alone historically has proven inadequate and, in the 20th
century, quality management moved successively through statistical
quality control, quality assurance, and strategic quality
management. This is the process approach to quality practices; it is
a management strategy that realizes that outputs are inextricably
linked to inputs and transformations. The specific processes used
by medical device development organizations can help estimate
their mechatronics maturity ... and that is a predictor of new product
development (NPD) quality, which is one reason why the FDA
believes that quality system management audits have value.
Mechatronic medical devices are the result of integration of the
engineering work of various HW disciplines (mechanical, electrical,
etc.), various SW disciplines (embedded, application, etc.), and - of
necessity, as many are now beginning to realize - various HF&E
disciplines (micro-, meso-, macro-, and mega-ergonomics; please
refer to diagram for definition of terms). Please also refer to middle
and lower figures on the right. Practitioners of these various
disciplines speak different technical languages and have very
different perspectives of the engineering process; in many cases,
some of the SW and HF&E practitioners have no engineering
background. This creates significant engineering management
problems that are typically not resolved with standard project
management methods. Some pretend that these are problems with
the HW, SW, and HF&E practitioners; in fact, they are engineering
management problems!

Permitting HW, SW, and HF&E work to proceed un-integrated in
individual "silos" is a regressive engineering management
approach that thwarts efficient communication & coordination,
thwarts the effective implementation of design controls, and
invariably reduces quality of the developed product, process, or
service - stifling innovation.
Why should mechatronics maturity be of concern to you? It
directly impacts economic, technical, and operational risk. NPD
presents a set of engineering management trade offs among the
four basic NPD attributes: budget, schedule, scope, and quality.
A low maturity NPD process is more costly and less time
efficient; trading off scope and quality against budget and
schedule will reduce market share and increases future liability.
Development processes that have traded off quality in favor of
budget and/or schedule invariably yield devices that suffer a
greater incidence of safety and effectiveness problems; some
can be the source of significant corporate liability. A high
maturity NPD process is quite cost and time efficient, permitting
the developing organization to focus on maximizing scope and
quality … and market share.






























